Opportunity
Green hydrogen is crucial for clean energy and deep decarbonization in industries, offering zero carbon emissions when used as a feedstock or fuel. However, 95% of hydrogen is currently produced through fossil fuel steam reforming, costing only $1.34-2.27 per kilogram but releasing more carbon species. As a cleaner alternative, electrolysis of water with renewable energy sources provides high-purity green hydrogen without carbon dioxide emission, but its high power consumption and cost ($4.15-23.27 per kilogram) hinder the development of hydrogen energy. Inefficiency and expensive existing catalyst materials for water electrolysis (precious metals e.g., Pt, IrO2) contribute to these challenges. Therefore, seeking a new catalyst for water electrolysis is essential to improve efficiency and reduce costs, paving the way for green hydrogen advancement.
Technology
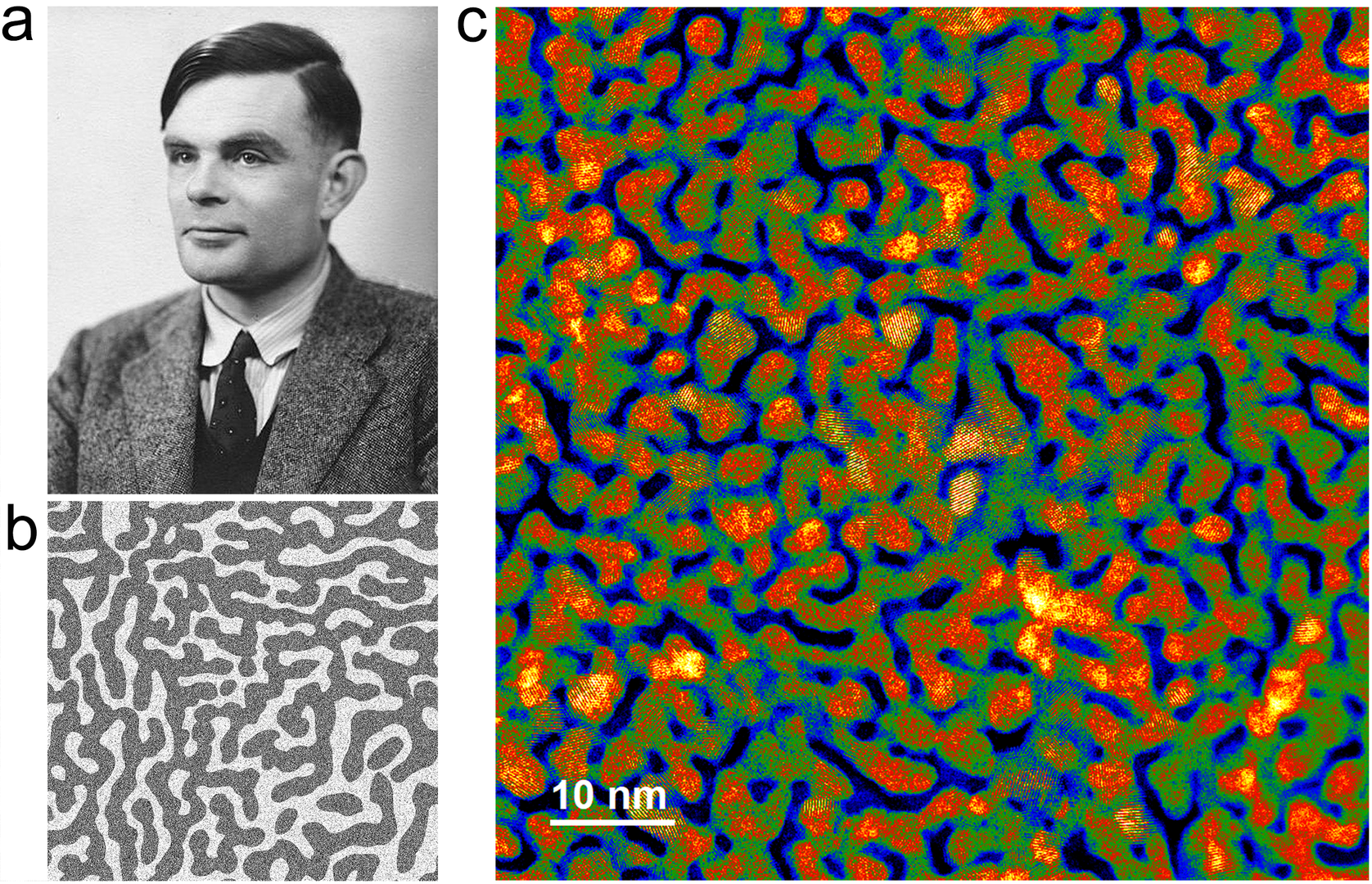
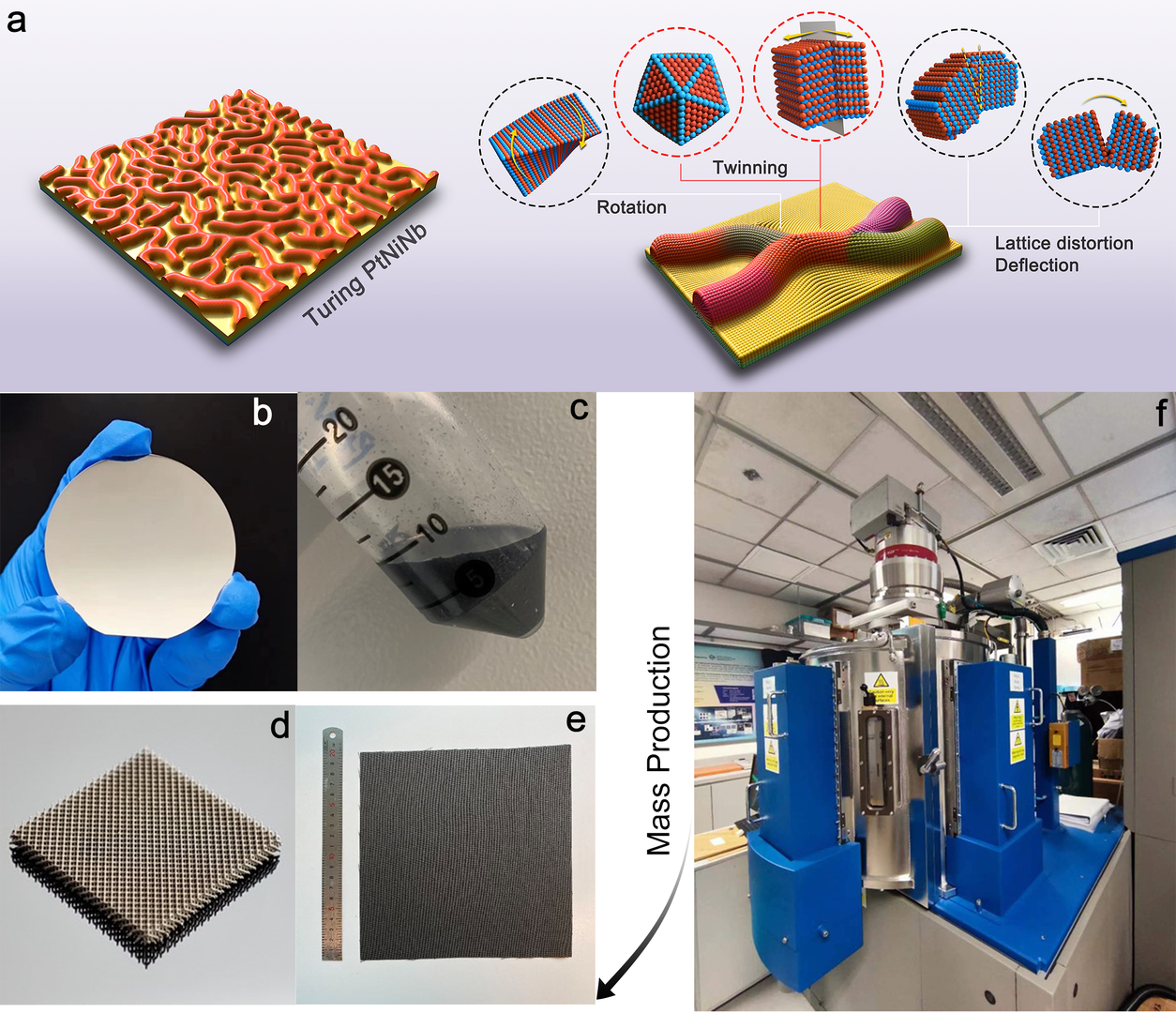
The idea of our Turing catalyst development is to utilize facile physical vapor deposition technology to precisely modulate the unique Turing nano-configuration to form nanoscale structures favorable for catalytic reactions. Turing catalysts have the characteristics of highly stable nanotwin networks and large lattice strains at the microscopic scale. This Turing structure accelerates electron transfer on the catalyst's surface, lowers the energy barrier of the water-splitting reaction, thus essentially improving the efficiency of water electrolysis and realizing the order-of-magnitude enhancement of catalytic performance. The electricity consumption of Turing-catalyst-powered water electrolyser stacks for large-scale hydrogen production can be reduced from 5.4 to 4.0 kWh/Nm3 H2, which greatly lower the cost of H2 products and contribute to the development of green-hydrogen industry. The Turing catalyst possesses the characteristics of simple fabrication methods, easy scalability, and diverse supporting available, enabling rapid realization of large-scale production above 100 kg/year.
Advantages
-
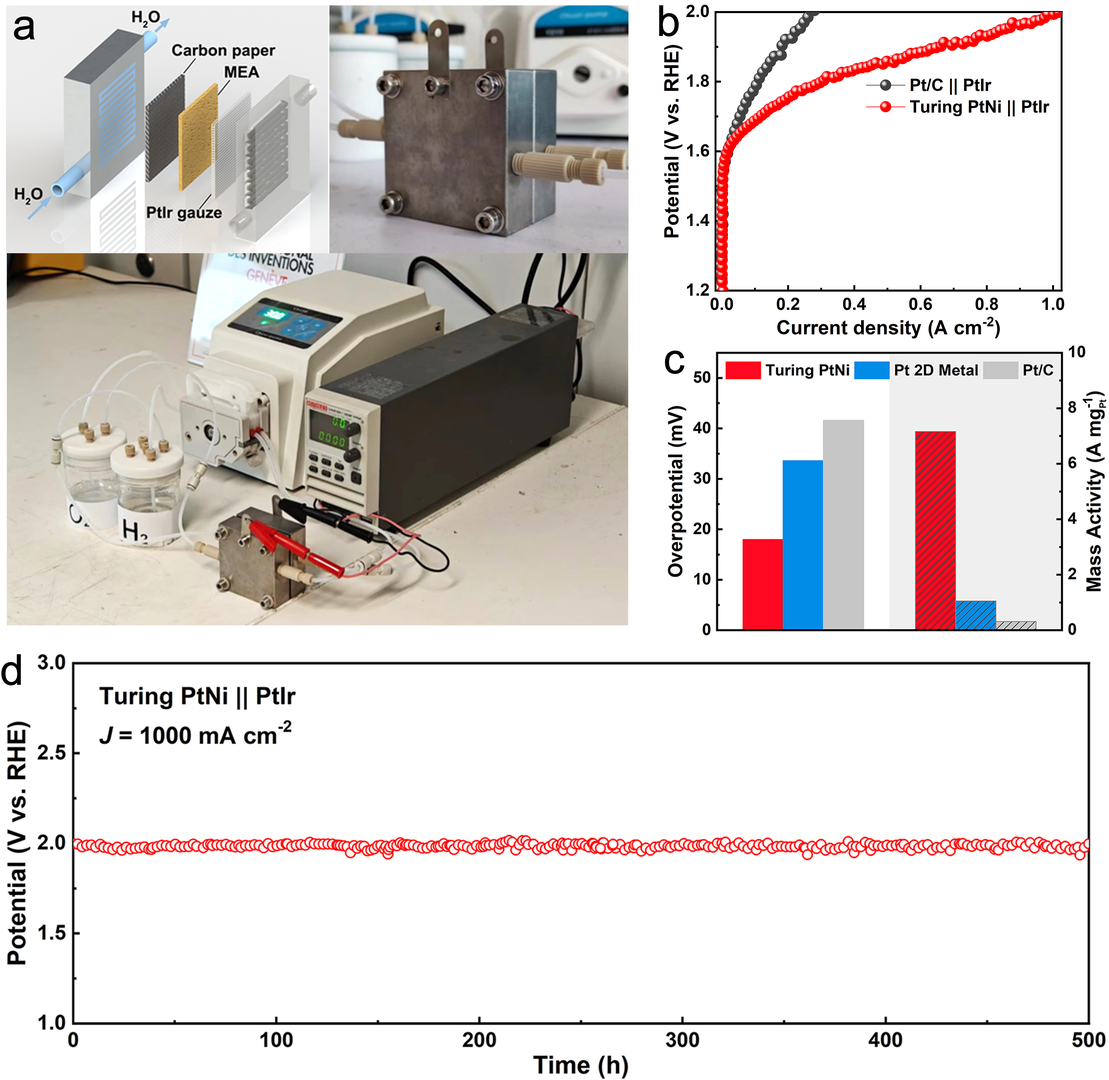
The mass activity of Turing PtNiNb is 23 times that of commercial Pt/C catalyst for catalyzing water splitting in anion/proton exchange membrane water electrolyzer.
-
The Turing catalyst is highly stable, the Turing catalyst based water electrolyzer can be stably operated over 500 h at 1000 mA/cm.
-
Diverse catalyst-form available. The Turing catalyst can be prepared as powder, catalyst ink, and can be deposited directly on carbon-supporting materials, metallic nets as well as metallic foams.
-
The Turing catalyst possesses the characteristics of simple fabrication methods and easy scalability, enabling rapid realization of large-scale production above 100 kg/year.
-
Turing catalysts exhibit high controllability of structure and composition, allowing for continuous product upgrades to meet the ever-changing market demands and competition.
Applications
- Turing catalysts as cathode and anode catalysts for hydrogen production by water electrolyzers, including alkaline electrolyzer, anion exchange membrane electrolyzer and proton exchange membrane electrolyzer.
- Providing PEM/AEM electrolyzer manufacturers with more efficient and inexpensive Turing catalysts as catalytic layer materials.
- Offering catalyst materials for the conversion of excess electricity from wind and solar energy into hydrogen storage energy.